General Description
Why "Bioorganic Chemistry"? It is because the main scientific interest of the group, that is organic synthesis, has always been intertwined with biological applications and biological means. Thus the group has synthesized biologically active molecules since 1983 (among them particularly noteworthy are beta-lactam antibiotics and enediynes) and has used biological tools (enzymes-microorganisms) for organic synthesis since 1985. The group is strongly active also in the synthesis of Active Pharmaceutical Ingredients. In the last 20 years the group has been deeply involved in diversity-oriented synthesis of drug-like molecules (in particular novel heterocycles). Towards that goal we have thoroughly used multicomponent reactions (but also solid-phase combinatorial synthesis and flow chemistry). Recently, the group is becoming more and more interested in the chemical contribution to Bioeconomy.
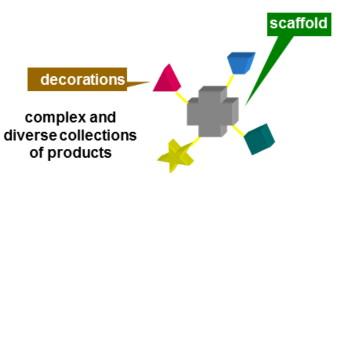
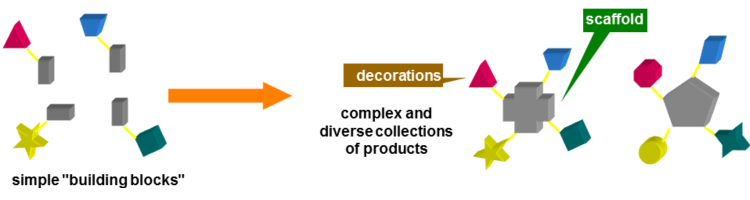
During the last 17 years, the group has been mainly active in Diversity Oriented Synthesis of libraries of drug-like molecules, especially using Multicomponent reactions (MCRs). They are, for their intimate nature, extremely convergent, producing a remarkably high increase of molecular complexity in just one step. Therefore, especially when the components may be varied at will, they are very well suited for the generation of libraries. Among MCRs, those based on the peculiar reactivity of isocyanides, such as the Ugi and the Passerini reactions or their modifications, have been among the most widely used, also in an industrial context.
Apart from that, the group has a 360° experience in organic synthesis (e.g. stereoselective synthesis, organocatalysis, biocatalysis, heterocycle synthesis and so on). Due to this well reknown experience in synthetic chemistry the BOG group is also involved in custom organic synthesis both of libraries and of APIs. We have always collaborated with private companies and are always happy to share our expertise with the industrial world.

Recently the group is also active in new fields. In particular flow chemistry and photoactivated reactions. You can see a video on photoredox reactons here:
https://www.youtube.com/watch?v=TQAWezfy7Lw&t
Contrary to what typically happens elsewhere, our group is formed by more than one professor. This is because we strongly believe that joining our efforts will allow to reach a sufficient critical mass. Thus, while each professor has his/her own independent research programmes, we share facilities, reagents, instruments, intermediates and discuss weekly in our group meetings the results of our research. In this way, our students can come in contact with several research projects, learning much more than what directly regards their own programme.
Our group is strongly committed to internationalization, and several of our students (both at master and Ph.D. level) carry out their theses through secondment periods in other european countries.
Finally, the BOG group is involved in dissemination initiatives, such as the laboratory "Alimenti a contatto" (organized inside the 2017 edition of Science Festival in Genova - october 26 - november 5), the Mole Day, the laboratory "Naturalmente polifenoli" (organized inside the 2017 edition of Science Festival in Genova),
Group Composition
Contacts
Contact Luca Banfi: E-mail: banfi@chimica.unige.it
Recent publications
- Moni, L., Basso, A.; Banfi, L.; Mori, A.; Risso, F.; Riva, R.; Lambruschini, C., "A thorough study on the photoisomerization of ferulic acid derivatives", Eur. J. Org. Chem. 2021, published online. DOI: 10.1002/ejoc.202100064
- Lambruschini, C., Demori, I., El Rashed, Z., Rovegno, L., Canessa, E., Cortese, K., Grasselli, E. and Moni, L., "Synthesis, Photoisomerization, Antioxidant Activity, and Lipid-Lowering Effect of Ferulic Acid and Feruloyl Amides", Molecules 2021, 26
- Zhang, B. W., Goldoni, L., Lambruschini, C., Moni, L., Imran, M., Pianetti, A., Pinchetti, V., Brovelli, S., De Trizio, L. and Manna, L., "Stable and Size Tunable CsPbBr3 Nanocrystals Synthesized with Oleylphosphonic Acid", Nano Letters 2020, 20, 8847-8853
- Pinna, A., Basso, A., Lambruschini, C., Moni, L., Riva, R., Rocca, V. and Banfi, L., "Stereodivergent access to all four stereoisomers of chiral tetrahydrobenzo f 1,4 oxazepines, through highly diastereoselective multicomponent Ugi-Joullie reaction", Rsc Advances 2020, 10, 965-972
- Moni, L., Banfi, L., Cartagenova, D., Cavalli, A., Lambruschini, C., Martino, E., Orru, R. V. A., Ruijter, E., Saya, J. M., Sgrignani, J. and Riva, R., "Zinc(ii)-mediated diastereoselective Passerini reactions of biocatalytically desymmetrised renewable inputs", Organic Chemistry Frontiers 2020, 7, 380-398
- Maffeis, V., Moni, L., Di Stefano, D., Giordani, S. and Riva, R., "Diversity-oriented synthesis of blue emissive nitrogen heterocycles and their conjugation with carbon nano-onions", Frontiers of Chemical Science and Engineering 2020, 14, 76-89
- Lanteri, D., Quattrosoldi, S., Soccio, M., Basso, A., Cavallo, D., Munari, A., Riva, R., Lotti, N. and Moni, L., "Regioselective Photooxidation of Citronellol: A Way to Monomers for Functionalized Bio-Polyesters", Frontiers in Chemistry 2020, 8,
- Lambruschini, C., Moni, L. and Banfi, L., "Diastereoselectivity in Passerini Reactions of Chiral Aldehydes and in Ugi Reactions of Chiral Cyclic Imines", European Journal of Organic Chemistry 2020, 2020, 3766-3778
- Vitali Forconesi, G., Banfi, L., Basso, A., Lambruschini, C., Moni, L. and Riva, R., "Synthesis of Polyoxygenated Heterocycles by Diastereoselective Functionalization of a Bio-Based Chiral Aldehyde Exploiting the Passerini Reaction", Molecules 2020, 25
- Capurro, P., Bergamaschi, E., Basso, A. and Moni, L., "An unexpected benzylic oxidation in the multicomponent synthesis of simplified analogs of anchinopeptolides and eusynstyelamides", Chemistry of Heterocyclic Compounds 2020, 56, 467-472
- Tomaselli, S., La Vitola, P., Pagano, K., Brandi, E., Santamaria, G., Galante, D., D'Arrigo, C., Moni, L., Lambruschini, C., Banfi, L., Lucchetti, J., Fracasso, C., Molinari, H., Forloni, G., Balducci, C. and Ragona, L., "Biophysical and in Vivo Studies Identify a New Natural-Based Polyphenol, Counteracting A beta Oligomerization in Vitro and A beta Oligomer-Mediated Memory Impairment and Neuroinflammation in an Acute Mouse Model of Alzheimer's Disease", Acs Chemical Neuroscience 2019, 10, 4462-4475
- Galante, D., Banfi, L., Baruzzo, G., Basso, A., D'Arrigo, C., Lunaccio, D., Moni, L., Riva, R. and Lambruschini, C., "Multicomponent Synthesis of Polyphenols and Their In Vitro Evaluation as Potential beta-Amyloid Aggregation Inhibitors", Molecules 2019, 24
- Bergamaschi, E., Capurro, P., Lambruschini, C., Riva, R. and Basso, A., "Stereoselective Synthesis of 3,5-Dihydroxypyrrolidin-2-ones Through a Photoinduced Multicomponent Reaction Followed by Dimerization", European Journal of Organic Chemistry 2019, 5992-5997
- Basso, A., Park, S. B. and Moni, L., "Editorial: Diversity Oriented Synthesis", Frontiers in Chemistry 2019, 6
- Banfi, L., Lambruschini, C., Moni, L. and Riva, R., "Renewable Starting Materials, Biocatalysis, and Multicomponent Reactions: A Powerful Trio for the Green Synthesis of Highly Valued Chemicals", Green Synthetic Processes and Procedures 2019, 61, 115-140
- Speich, E., Banfi, L., Moni, L., Riva, R., Rocca, V. and Basso, A., "Zr-mediated synthesis of chiral cyclic imines and their application in Betti reactions", Chemistry of Heterocyclic Compounds 2018, 54, 329-333
- Moni, L., De Moliner, F., Garbarino, S., Saupe, J., Mang, C. and Basso, A., "Exploitation of the Ugi 5-Center-4-Component Reaction (U-5C-4CR) for the Generation of Diverse Libraries of Polycyclic (Spiro) Compounds", Frontiers in Chemistry 2018, 6, 369
- Lambruschini, C., Villa, S., Banfi, L., Canepa, F., Morana, F., Relini, A., Riani, P., Riva, R. and Silvetti, F., "Enzymatically promoted release of organic molecules linked to magnetic nanoparticles", Beilstein Journal of Nanotechnology 2018, 9, 986-999
- Lambruschini, C., Basso, A., Moni, L., Pinna, A., Riva, R. and Banfi, L., "Bicyclic Heterocycles from Levulinic Acid through a Fast and Operationally Simple Diversity-Oriented Multicomponent Approach", European Journal of Organic Chemistry 2018, 2018, 5445-5455
- Ibba, F., Capurro, P., Garbarino, S., Anselmo, M., Moni, L. and Basso, A., "Photoinduced Multicomponent Synthesis of alpha-Silyloxy Acrylamides, an Unexplored Class of Silyl Enol Ethers", Organic Letters 2018, 20, 1098-1101
- Giustiniano, M., Moni, L., Sangaletti, L., Pelliccia, S., Basso, A., Novellino, E. and Tron, G. C., "Interrupted Ugi and Passerini Reactions: An Underexplored Treasure Island", Synthesis-Stuttgart 2018, 50, 3549-3570
- Capurro, P., Moni, L., Galatini, A., Mang, C. and Basso, A., "Multi-Gram Synthesis of Enantiopure 1,5-Disubstituted Tetrazoles Via Ugi-Azide 3-Component Reaction", Molecules 2018, 23
- Basso, A., Chiorri, C., Bracco, F., Carnasciali, M. M., Alloisio, M. and Grotti, M., "Improving the interest of high-school students toward chemistry by crime scene investigation", Chemistry Education Research and Practice 2018, 19, 558-566.
- Lambruschini, C.; Basso, A.; Banfi, L., Integrating biocatalysis and multicomponent reactions, Drug Discovery Today: Technologies, 2018, 29, 3-9. DOI: 10.1016/j.ddtec.2018.06.004
- Lambruschini, C.; Galante, D.; Moni, L.; Ferraro, F.; Gancia, G.; Riva, R.; Traverso, A.; Banfi L.; D'Arrigo, C., Multicomponent, Fragment-Based, Synthesis of Polyphenol-containing Peptidomimetics and their Inhibiting Activity on BetaAmyloid Oligomerization, Org. Biomol. Chem., 2017, 15, 9331-9351. DOI: 10.1039/C7OB02182H. DOI: 10.1039/C7OB02182H.
- Caputo, S.; Banfi, L.; Basso, A.; Galatini, A.; Moni, L.; Riva, R.; Lambruschini, C. Diversity-Oriented Synthesis of Various Enantiopure Heterocycles by Coupling Organocatalysis with Multicomponent Reactions, European Journal of Organic Chemistry, 2017, 6619-6628.
- Anselmo, M.; Moni, L.; Ismail, H.; Comoretto, D.; Riva, R.; Basso, A., Photocatalyzed synthesis of isochromanones and isobenzofuranones under batch and flow conditions, Beilstein Journal of Organic Chemistry 2017, 13, 1456-1462.
- Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. P., To each his own: isonitriles for all flavors. Functionalized isocyanides as valuable tools in organic synthesis, Chemical Society Reviews, 2017, 46, 1295-1357.
- Banfi, L.; Basso, A.; Lambruschini, C.; Moni, L.; Riva, R., Synthesis of Seven-membered Nitrogen Heterocycles through the Ugi Multicomponent Reaction, Chemistry of Heterocyclic Compounds, 2017, 53, 382.
- Virgone-Carlotta, A.; Dufour, E.; Bacot, S.; Ahmadi, M.; Cornou, M.; Moni, L.; Garcia, J.; Chierici, S.; Garin, D.; Marti-Batlle, D.; Perret, P.; Ghersi-Egea, J. F.; Sallanon, M. M.; Fagret, D.; Ghezzi, C., New diketopiperazines as vectors for peptide protection and brain delivery: Synthesis and biological evaluation, Journal of Labelled Compounds & Radiopharmaceuticals, 2016, 59, 517-530.
- Veronesi, M.; Giacomina, F.; Romeo, E.; Castellani, B.; Ottonello, G.; Lambruschini, C.; Garau, G.; Scarpelli, R.; Bandiera, T.; Piomelli, D.; Dalvit, C., Fluorine nuclear magnetic resonance-based assay in living mammalian cells, Analytical Biochemistry, 2016, 495, 52-59.
- Spallarossa, M.; Banfi, L.; Basso, A.; Moni, L.; Riva, R., Access to Polycyclic Alkaloid-Like Structures by Coupling the Passerini and Ugi Reactions with Two Sequential Metal-Catalyzed Cyclizations, Advanced Synthesis & Catalysis, 2016, 358, 2940-2948.
- Moni, L.; Gers-Panther, C. F.; Anselmo, M.; Muller, T. J. J.; Riva, R., Highly Convergent Synthesis of Intensively Blue Emissive Furo 2,3-c isoquinolines by a Palladium-Catalyzed Cyclization Cascade of Unsaturated Ugi Products, Chemistry-a European Journal, 2016, 22, 2020-2031.
- Spallarossa, M.; Wang, Q.; Riva, R.; Zhu, J. P., Synthesis of Vinyl Isocyanides and Development of a Convertible Isonitrile, Organic Letters, 2016, 18, 1622-1625.
- Moni, L.; Banfi, L.; Riva, R.; Basso, A., External-Oxidant-Based Multicomponent Reactions, Synthesis-Stuttgart, 2016, 48, 4050-4059.
- Moni, L.; Banfi, L.; Basso, A.; Martino, E.; Riva, R., Diastereoselective Passerini Reaction of Biobased Chiral Aldehydes: Divergent Synthesis of Various Polyfunctionalized Heterocycles, Organic Letters, 2016, 18, 1638-1641. Errata corrige: p. 3306.
- Moni, L.; Banfi, L.; Basso, A.; Bozzano, A.; Spallarossa, M.; Wessjohann, L.; Riva, R., Passerini Reactions on Biocatalytically Derived Chiral Azetidines, Molecules, 2016, 21.
- Lambruschini, C.; Banfi, L.; Guanti, G., Switching the Photochromic Activity of Acenaphthylene Derivatives through a Tandem Nucleophile-Promoted Addition Reaction, Chemistry-a European Journal, 2016, 22, 13831-13834.
- Garbarino, S.; Ravelli, D.; Protti, S.; Basso, A., Photoinduced Multicomponent Reactions, Angewandte Chemie-International Edition, 2016, 55, 15476-15484.
- Cini, E.; Banfi, L.; Barreca, G.; Carcone, L.; Malpezzi, L.; Manetti, F.; Marras, G.; Rasparini, M.; Riva, R.; Roseblade, S.; Russo, A.; Taddei, M.; Vitale, R.; Zanotti-Gerosa, A., Convergent Synthesis of the Renin Inhibitor Aliskiren Based on C5-C6 Disconnection and CO2H-NH2 Equivalence, Organic Process Research & Development, 2016, 20, 270-283.
- Caputo, S.; Basso, A.; Moni, L.; Riva, R.; Rocca, V.; Banfi, L., Diastereoselective Ugi reaction of chiral 1,3-aminoalcohols derived from an organocatalytic Mannich reaction, Beilstein Journal of Organic Chemistry, 2016, 12, 139-143.
- Tassano, E.; Alama, A.; Basso, A.; Dondo, G.; Galatini, A.; Riva, R.; Banfi, L., Conjugation of Hydroxytyrosol with Other Natural Phenolic Fragments: From Waste to Antioxidants and Antitumour Compounds, European Journal of Organic Chemistry, 2015, 6710-6726.
- Moni, L.; Denissen, M.; Valentini, G.; Muller, T. J. J.; Riva, R., Diversity-Oriented Synthesis of Intensively Blue Emissive 3-Hydroxyisoquinolines by Sequential Ugi Four-Component Reaction/Reductive Heck Cyclization, Chemistry-a European Journal, 2015, 21, 753-762.
- Moni, L.; Banfi, L.; Basso, A.; Carcone, L.; Rasparini, M.; Riva, R., Ugi and Passerini Reactions of Biocatalytically Derived Chiral Aldehydes: Application to the Synthesis of Bicyclic Pyrrolidines and of Antiviral Agent Telaprevir, Journal of Organic Chemistry, 2015, 80, 3411-3428.
- Gardella, L.; Basso, A.; Prato, M.; Monticelli, O., On stereocomplexed polylactide materials as support for PAMAM dendrimers: synthesis and properties, Rsc Advances, 2015, 5, 46774-46784.
- Garbarino, S.; Protti, S.; Basso, A., Toward a Green Atom Economy: Development of a Sustainable Multicomponent Reaction, Synthesis-Stuttgart, 2015, 47, 2385-2390.
- Terol, A.; Ardini, F.; Basso, A.; Grotti, M., Determination of selenium urinary metabolites by high temperature liquid chromatography-inductively coupled plasma mass spectrometry, Journal of Chromatography A, 2015, 1380, 112-119.
- Veronesi, M.; Romeo, E.; Lambruschini, C.; Piomelli, D.; Bandiera, T.; Scarpelli, R.; Garau, G.; Dalvit, C., Fluorine NMR-Based Screening on Cell Membrane Extracts, Chemmedchem, 2014, 9, 286-289.
- Monticelli, O.; Putti, M.; Gardella, L.; Cavallo, D.; Basso, A.; Prato, M.; Nitti, S., New Stereocomplex PLA-Based Fibers: Effect of POSS on Polymer Functionalization and Properties, Macromolecules, 2014, 47, 4718-4727.
- Moni, L.; Banfi, L.; Basso, A.; Galatini, A.; Spallarossa, M.; Riva, R., Enantio- and Diastereoselective Synthesis of Highly Substituted Benzazepines by a Multicomponent Strategy Coupled with Organocatalytic and Enzymatic Procedures, Journal of Organic Chemistry, 2014, 79, 339-351.
- Moni, L.; Banfi, L.; Basso, A.; Brambilla, A.; Riva, R., Diversity-oriented synthesis of dihydrobenz-oxazepinones by coupling the Ugi multicomponent reaction with a Mitsunobu cyclization, Beilstein Journal of Organic Chemistry, 2014, 10, 209-212.
- Garbarino, S.; Banfi, L.; Riva, R.; Basso, A., Three in the Spotlight: Photoinduced Stereoselective Synthesis of (Z)-Acyloxyacrylamides through a Multicomponent Approach, Journal of Organic Chemistry, 2014, 79, 3615-3622.
- Dufour, E.; Moni, L.; Bonnat, L.; Chierici, S.; Garcia, J., 'Clickable' 2,5-diketopiperazines as scaffolds for ligation of biomolecules: their use in A beta inhibitor assembly, Organic & Biomolecular Chemistry, 2014, 12, 4964-4974.
- De Moliner, F.; Bigatti, M.; De Rosa, C.; Banfi, L.; Riva, R.; Basso, A., Synthesis of triazolo-fused benzoxazepines and benzoxazepinones via Passerini reactions followed by 1,3-dipolar cycloadditions, Molecular Diversity, 2014, 18, 473-482.
- De Moliner, F.; Bigatti, M.; Banfi, L.; Riva, R.; Basso, A., OPHA (Oxidation-Passerini-Hydrolysis-Alkylation) Strategy: a Four-Step, One-Pot Improvement of the Alkylative Passerini Reaction, Organic Letters, 2014, 16, 2280-2283.
- Banfi, L.; Basso, A.; Moni, L.; Riva, R., The Alternative Route to Enantiopure Multicomponent Reaction Products: Biocatalytic or Organocatalytic Enantioselective Production of Inputs for Multicomponent Reactions, European Journal of Organic Chemistry, 2014, 2014, 2005-2015.
- Morana, F.; Basso, A.; Riva, R.; Rocca, V.; Banfi, L., The homo-PADAM Protocol: Stereoselective and Operationally Simple Synthesis of alpha-Oxo- or alpha-Hydroxy-gamma-acylaminoamides and Chromanes, Chemistry-a European Journal, 2013, 19, 4563-4569.
- Moni, L.; Marra, A.; Skotnicki, J. S.; Koehn, F. E.; Abou-Gharbia, M.; Dondoni, A., Synthesis of rapamycin glycoconjugates via a CuAAC-based approach, Tetrahedron Letters, 2013, 54, 6999-7003.
- Lambruschini, C.; Veronesi, M.; Romeo, E.; Garau, G.; Bandiera, T.; Piomelli, D.; Scarpelli, R.; Dalvit, C., Development of Fragment-Based n-FABS NMR Screening Applied to the Membrane Enzyme FAAH, Chembiochem, 2013, 14, 1611-1619.
- Gardella, L.; Basso, A.; Prato, M.; Monticelli, O., PLA/POSS Nanofibers: A Novel System for the Immobilization of Metal Nanoparticles, Acs Applied Materials & Interfaces, 2013, 5, 7688-7692.
- Bertolacci, L.; Romeo, E.; Veronesi, M.; Magotti, P.; Albani, C.; Dionisi, M.; Lambruschini, C.; Scarpelli, R.; Cavalli, A.; De Vivo, M.; Piomelli, D.; Garau, G., A Binding Site for Nonsteroidal Anti-inflammatory Drugs in Fatty Acid Amide Hydrolase, Journal of the American Chemical Society, 2013, 135, 22-25.
- Basso, A.; Banfi, L.; Garbarino, S.; Riva, R., Ketene Three-Component Reaction: A Metal-Free Multicomponent Approach to Stereodefined Captodative Olefins, Angewandte Chemie-International Edition, 2013, 52, 2096-2099.
- Banfi, L.; Bagno, A.; Basso, A.; De Santis, C.; Riva, R.; Rastrelli, F., Long-Range Diastereoselectivity in an Ugi Reaction: Stereocontrolled and Diversity-Oriented Synthesis of Tetrahydrobenzoxazepines, European Journal of Organic Chemistry, 2013, 2013, 5064-5075.
- Sonaglia, L.; Banfi, L.; Riva, R.; Basso, A., Multicomponent approach to the alkaloid-type 2-aza-7-oxabicyclo 4.3.0 nonane framework, Tetrahedron Letters, 2012, 53, 6516-6518.
- Morana, F.; Basso, A.; Bella, M.; Riva, R.; Banfi, L., Organocatalytic Asymmetric Synthesis of beta-Aryl-beta-isocyano Esters, Advanced Synthesis & Catalysis, 2012, 354, 2199-2210.
- Cerulli, V.; Banfi, L.; Basso, A.; Rocca, V.; Riva, R., Diversity oriented and chemoenzymatic synthesis of densely functionalized pyrrolidines through a highly diastereoselective Ugi multicomponent reaction, Organic & Biomolecular Chemistry, 2012, 10, 1255-1274.
- Bisignano, P.; Lambruschini, C.; Bicego, M.; Murino, V.; Favia, A. D.; Cavalli, A., In Silico Deconstruction of ATP-Competitive Inhibitors of Glycogen Synthase Kinase-3 beta, Journal of Chemical Information and Modeling, 2012, 52, 3233-3244.
- Banfi, L.; Basso, A.; Chiappe, C.; De Moliner, F.; Riva, R.; Sonaglia, L., Development of a stereoselective Ugi reaction starting from an oxanorbornene beta-amino acid derivative, Organic & Biomolecular Chemistry, 2012, 10, 3819-3829.
- Riva, R.; Banfi, L.; Castaldi, G.; Ghislieri, D.; Malpezzi, L.; Musumeci, F.; Tufaro, R.; Rasparini, M., Selective Chemical Oxidation of Risperidone: A Straightforward and Cost-Effective Synthesis of Paliperidone, European Journal of Organic Chemistry, 2011, 2319-2325.
- Riva, R.; Banfi, L.; Basso, A.; Zito, P., A new diversity oriented and metal-free approach to highly functionalized 3H-pyrimidin-4-ones, Organic & Biomolecular Chemistry, 2011, 9, 2107-2122.
- De Moliner, F.; Crosignani, S.; Galatini, A.; Riva, R.; Basso, A., Novel Application of alpha-Azido Aldehydes in Multicomponent Reactions: Synthesis of Triazolo-Fused Dihydrooxazinones via a Passerini Reaction-Dipolar Cycloaddition Strategy, Acs Combinatorial Science, 2011, 13, 453-457.
- De Moliner, F.; Banfi, L.; Riva, R.; Basso, A., Beyond Ugi and Passerini Reactions: Multicomponent Approaches Based on Isocyanides and Alkynes as an Efficient Tool for Diversity Oriented Synthesis, Combinatorial Chemistry & High Throughput Screening, 2011, 14, 782-810.
- Basso, A.; Banfi, L.; Riva, R., Divergent Synthesis of Novel Five-Membered Heterocyclic Compounds by Base-Mediated Rearrangement of Acrylamides Derived from a Novel Isocyanide-Based Multicomponent Reaction, Molecules, 2011, 16, 8775-8787.
- Basso, A.; Banfi, L.; Guanti, G.; Riva, R.; Tosatti, P., Elaboration of Peptidomimetics Derived from a PADAM Approach: Synthesis of Polyfunctionalised 2(1H)-Pyrazinones via an Unexpected Aromatisation, Synlett, 2011, 2009-2012.
- Banfi, L.; Basso, A.; Giardini, L.; Riva, R.; Rocca, V.; Guanti, G., Tandem Ugi MCR/Mitsunobu Cyclization as a Short, Protecting-Group-Free Route to Benzoxazinones with Four Diversity Points, European Journal of Organic Chemistry, 2011, 100-109.
- Banfi, L.; Basso, A.; Cerulli, V.; Rocca, V.; Riva, R., Long-range diastereoselectivity in Ugi reactions of 2-substituted dihydrobenzoxazepines, Beilstein Journal of Organic Chemistry, 2011, 7, 976-979.
- Appendino, G.; Banfi, L., Molecular diversity and natural products, Molecular Diversity, 2011, 15, 291-292.
- Riva, R.; Banfi, L.; Basso, A.; Gandolfo, V.; Guanti, G., Enzymatically Asymmetrised Chiral Building Blocks for the Synthesis of Complex Natural Product Analogues: The Synthesis of Dynemicin Analogues from 2-(Quinolin-4-yl)propane-1,3-diol, European Journal of Organic Chemistry, 2010, 2768-2787.
- Riva, R.; Banfi, L.; Basso, A.; Cerulli, V.; Guanti, G.; Pani, M., A Highly Convergent Synthesis of Tricyclic N-Heterocycles Coupling an Ugi Reaction with a Tandem S(N)2 '-Heck Double Cyclization, Journal of Organic Chemistry, 2010, 75, 5134-5143.
- Moni, L.; Rossetti, S.; Scoponi, M.; Marra, A.; Dondoni, A., Immobilization of calix 4 arene-based glycoclusters on TiO2 nanoparticles via click Cu(I)-catalyzed azide-alkyne coupling, Chemical Communications, 2010, 46, 475-477.
- Moni, L.; Ciogli, A.; D'Acquarica, I.; Dondoni, A.; Gasparrini, F.; Marra, A., Synthesis of Sugar-Based Silica Gels by Copper-Catalysed Azide-Alkyne Cycloaddition via a Single-Step Azido-Activated Silica Intermediate and the Use of the Gels in Hydrophilic Interaction Chromatography, Chemistry-a European Journal, 2010, 16, 5712-5722.
- Guanti, G.; Banfi, L.; Basso, A.; Bondanza, L.; Guglieri, G.; Powles, K.; Riva, R., Optimized synthesis of phosphatidylserine, Amino Acids, 2010, 39, 367-373.
- De Moliner, F.; Crosignani, S.; Banfi, L.; Riva, R.; Basso, A., Synthesis of 5-Carboxamide-oxazolines with a Passerini-Zhu/Staudinger-Aza-Wittig Two-Step Protocol, Journal of Combinatorial Chemistry, 2010, 12, 613-616.
- Basso, A.; Banfi, L.; Riva, R., A Marriage of Convenience: Combining the Power of Isocyanide-Based Multicomponent Reactions with the Versatility of (Hetero)norbornene Chemistry, European Journal of Organic Chemistry, 2010, 1831-1841.
- Basso, A.; Banfi, L.; Guanti, G.; Riva, R., Straightforward stereoselective synthesis of polyfunctionalized cyclohexenols using a multicomponent approach, Tetrahedron, 2010, 66, 2390-2397.
- Banfi, L.; Riva, R.; Basso, A., Coupling Isocyanide-Based Multicomponent Reactions with Aliphatic or Acyl Nucleophilic Substitution Processes, Synlett, 2010, 23-41.
- Banfi, L.; Basso, A.; Cerulli, V.; Guanti, G.; Lecinska, P.; Monfardini, I.; Riva, R., Multicomponent synthesis of dihydrobenzoxazepinones, bearing four diversity points, as potential alpha-helix mimics, Molecular Diversity, 2010, 14, 425-442.
- Banfi, L.; Basso, A.; Casuscelli, F.; Guanti, G.; Naz, F.; Riva, R.; Zito, P., Synthesis of Novel Isochromene Derivatives by Tandem Ugi Reaction/Nucleophilic Substitution, Synlett, 2010, 85-88.
News
- An interview to Lisa Moni regarding the BIODEST European project was recently published (see page 17).
- 23-3-2018: Elena Barisone, Daniele Cartagenova and Alberto Cherubin have obtained the master degree in Chemical Sciences.
- 15-12-2017: Pietro Capurro and Elena Speich have obtained the Master Degree in Chemical Sciences with 110/110 magna cum laude
- 20-10-2017: Chiara Lambruschini has given a lecture at the "Giornata della Chimica Ligure" and her presentation has been awarded the third prize as "best oral communication".

- 10-10-2017: two new papers have been accepted for publication on Eur.J.Org.Chem. and Org.Biomol.Chem.
- 29-9-2017: Hossny Ismail is leaving the group for (hopefully) a successful career elsewhere
- 29-9-2017: Manuel Anselmo is leaving for a secondment stay in Regensburg. He will be back in about 6 months.
- 21-7-2017: Marta Nola has obtained her master degree in Chemical Sciences with a mark of 108/110. Congratulations!
- 5-7-2017: Hossny Ismail has obtained his international master degree in SERP-CHEM. Congratulations!
- 16-6-2017: Giulio Less has obtained his master degree in Chemical Sciences with a mark of 106/110. Congratulations!
- 15-6-2017: a video describing a photochemical reaction has been published on YouTube
- 4-4-2017: Renata Riva and Andrea Basso have been habilitated for the role of full professor in Organic Chemistry. Congratulations!
- 27-3-2017: Sergio Mulone has obtained the master degree in Chemical Sciences Magna cum Laude.
- 21-3-2017: Luca Banfi has delivered an invited lecture at the minisymposium "Recent Advances in MCR Chemistry" hold in Groningen (NL). The title of the conference was "Multicomponent reactions starting from biobased components: a contribution to bioeconomy"
- 24-2-2017: Samantha Caputo has been awarded the Ph.D. title in Science and Technology of Chemistry and Materials, with a thesis entitled "Topics in isocyanide-based multicomponent reactions (IMCRs): scope, selectivity and synthetic access to complex heterocyclic scaffolds". The defense was carried out at the University of Genova. Congratulations to Samantha and best wishes for her new job at the Italian Institute of Technology.
- 10-2-2017: Alessandro Sannino has obtained the master degree in Chemical Sciences Magna cum Laude.
Collaborations
Ongoing external academic collaborations
- The BOG group is involved in an interdisciplinary project named "From bio-inspired molecules to Alzheimer's disease prevention" funded by Fondazione Cariplo. For this project it collaborates with C.N.R., Macromolecular Institute (Milan, Genova) (C. D'Arrigo, S. Tomaselli, L. Ragona) and the "Mario Negri" Pharmacological Institute (G. Forloni, C. Balducci).
- University of Duesseldorf (T.J.J. Mueller) for the synthesis of fluorescent dyes through one-pot multicomponent-based processes.
- Vrije Universiteit, Amsterdam (R. Orru, E. Ruijter) in a project involving coupling of biocatalysis with multicomponent reactions.
- Edimburgh University (M. Bradley) for the development of biological tools activated by palladium.
- IRCSS San Martino (M. Viale) in projects related to the development of new anti-cancer agents.
- Universidad de Valladolid (A. Barbero) for using silicon-based MCRs on biobased substrates.
- Università di Pavia (S. Protti) for photochemical multicomponent reactions.
- University of Torino - Italian Institute of Technology (S. Giordani): attachment of fluorescent probes to nanomaterials.
Ongoing inter-departmental academic collaborations
- F. Canepa, P. Riani: magnetic nanoparticles bound to enzymatically cleavable linkers.
- D. Cavallo: synthesis of new additives for polymers.
- M. Grotti: Analysis of selenium containing metabolites.
- O. Monticelli: new biopolymers.
Ongoing collaborations with private companies
- Active Cells
- Bio Industria
- Chemical Control
- Lamberti group
How to join the group
- If you are a master student at the university of Genova: there are open positions for your thesis in our group! Just contact us.
- If you are a master student of a non-italian european university: you can come to Genova through the Erasmus+ programme either under the "study" or "traineeship" type of mobility, and perform part of the thesis (typically 5-10 months) in our laboratories. We have several established agreements (University at Duesseldorf, Barcelona University, Groningen University, Gent University, Leuven University, Pierre at Marie Curie University, Burgos University, Valladolid University and several others stipulated by our colleagues). If you are interested, please contact us well in advance (at least 6 months before the start of the stage period). Ask us for more informations.
- If you are a prospective Ph.D. student, please note that you must apply in may-june 2018 for the doctorate starting on november 1, 2018. You can find the call and other informations at https://www.studenti.unige.it/postlaurea/dottorati/XXXIII/ENG/. Application is completely free. If you want to apply and desire to join our group, please let us know that before applying, since we can give you precious advice
- If you are going to get a master degree and want to join us through an Erasmus + programme for post-lauream traineeship, it is OK, but please note that we require at least a 6 months stage, possibly not in summer (in august our laboratory is closed).
- If you are a Ph.D. student in other universities and want to join us for a secondment period (at least 6 months) you are welcome, but please note that we have no possibility to give you any financial sponsorship. You need to be sponsored by our university or, in alternative, you can apply to the Erasmus programme or other inter-national funding schemes.
- If you are searching for a post-doc position this will be much more difficult. At the moment we do not have open positions. If in the future we will have some, they will be posted on this site. Otherwise, please do not contact us for post-doc positions.
Curricula
GABRIELLA VITALI FORCONESI
Gabriella Vitali Forconesi was born in Genoa in 1991. She received her Bachelor Degree in Chemistry and Chemical Technologies with honors in Genoa on March 2014 with a thesis on the synthesis of derivatives of hydroxytyrosol with a potential antitumor activity, under the supervision of Prof. Luca Banfi. In 2014 she started her master thesis in Genoa and she graduated in Chemical Science with the highest mark (110/110 cum laudae) on March 2016 with a final dissertation on the study of batch and flow chemical processes to access Active Pharmaceutical Ingredients. This research project was done in conjunction with a pharmaceutical industry (Bioindustria L.I.M. S.p.a., Fresonara (Al)) and under the supervision of Prof. Andrea Basso and Dr. Alberto Moro. In November 2016 she started her PhD in the BOG group under the supervision of Prof.ssa Renata Riva, working in the field of multicomponent reactions and biocatalysis. In particularly, she is working on stereoselective multicomponent reactions of biobased chiral aldehydes derived from chemoenzymatic strategies.
E-mail: gabriella.vitali.f@gmail.com
PIETRO CAPURRO
Pietro was born in 1993 and raised in Genoa. He attended the University of Genoa and received his Bachelor Degree in Chemistry with honors in October 2015, under the supervision of Prof. Andrea Basso (thesis: “Synthesis and processing of Ugi reaction’s products for a combinatorial library”). In December 2017 he received his Master Degree in Chemical Sciences with the highest marks (110/110 cum laude), having worked on a thesis concerning a novel photoinduced multicomponent synthesis of alpha-amidosilylenolethers and their synthetic application, project that led to a publication on Organic Letters (Jan 2018, http://pubs.acs.org/doi/full/10.1021/acs.orglett.8b00009); project supervisors were Prof. Andrea Basso and Luca Banfi (co-supervisor). He’s currently working as post-master fellow under the lead of Prof. Andrea Basso, in a project involving the use of chiral imines obtained through reduction of lactams with Schwrtz Reagent in Ugi multicomponent reactions, for the creation of a combinatorial library.
E-mail: pietro.capurro@outlook.it